
The more accurately we know one of these values, the less.
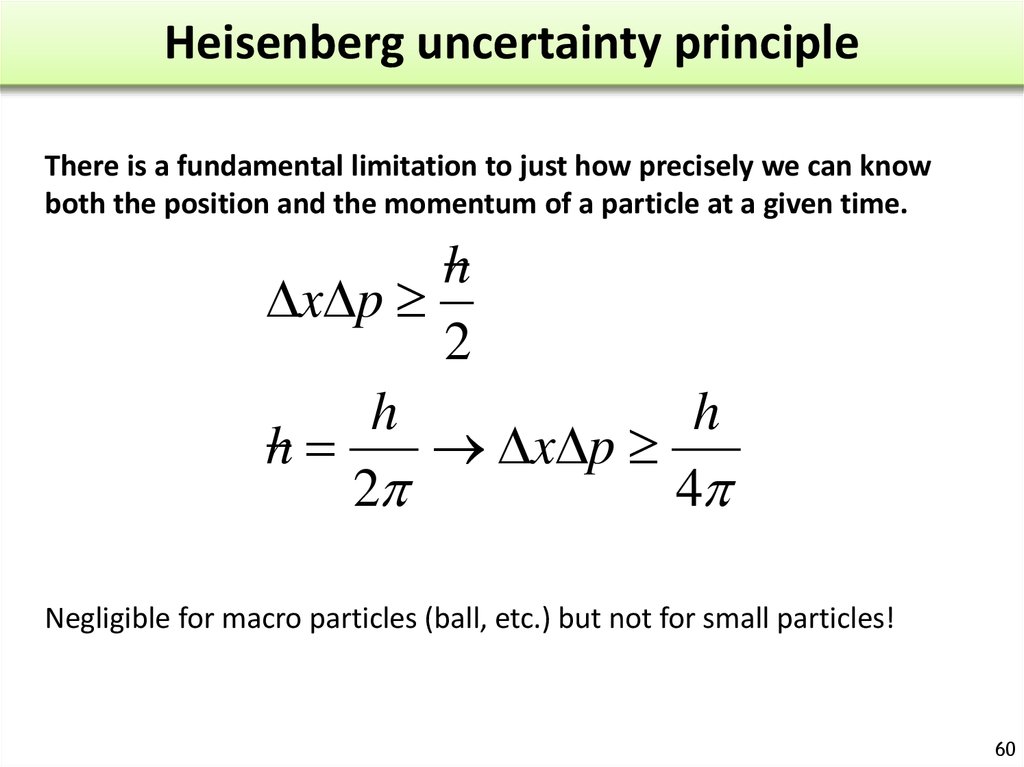
The finite lifetimes of these states can be deduced from the shapes of spectral lines observed in atomic emission spectra. The uncertainty principle says that we cannot measure the position (x) and the momentum (p) of a particle with absolute precision. The product of position and momentum uncertainty will always be larger than h. The Heisenberg uncertainty principle quantifies the limitations to which the position and momentum of a particle can be known at the same time. To illustrate, consider the excited states of an atom. The de Broglie equation depicts the relationship between a particle’s mass and velocity and its dependence on wavelength. The reason is that the frequency of a state is inversely proportional to time and the frequency connects with the energy of the state, so to measure the energy with good precision, the state must be observed for many cycles.

The story of quantum physics probably best begins with light. Wave-particle duality: light Light as a wave. The principle describes the precision with which these two quantities can be known for any object. Explore the Heisenberg uncertainty principle by calculating uncertainty in position given the uncertainty in momentum for Bohr model of hydrogen. These two quantities, position and momentum, are the subject of the Heisenberg uncertainty principle.
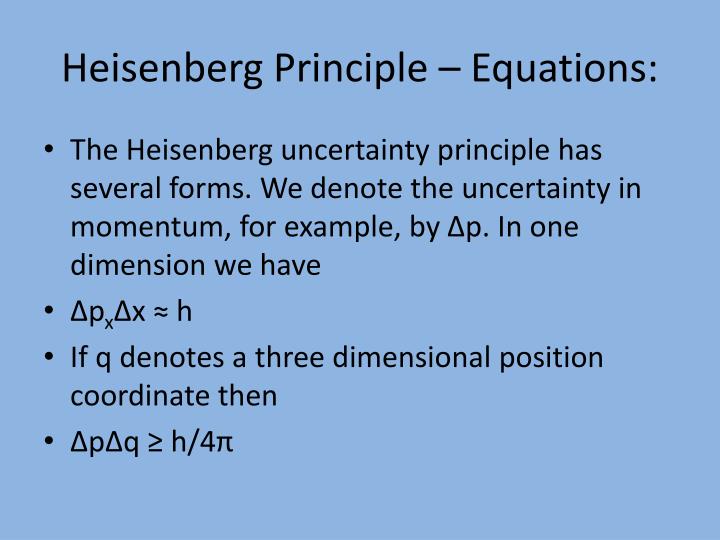
Nevertheless, the general meaning of the energy-time principle is that a quantum state that exists for only a short time cannot have a definite energy. The Heisenberg uncertainty principle states that there is a limit to how precisely certain pairs of physical properties of a particle can be known simultaneously. For technical reasons beyond this discussion.


 0 kommentar(er)
0 kommentar(er)
